40Hz Stimulation Shows Substantial Reduction in Tau Protein and Slowed Cognitive Decline in Late-Onset Alzheimer's Patients
GemPagesAlzheimer's disease (AD) still lacks effective long-term, non-invasive therapies. However, a recent study by a research team at MIT, published in Alzheimer's & Dementia, offers new hope. The study found that after two years of daily, at-home treatment with one hour of 40Hz audiovisual stimulation (GENUS therapy), three women with late-onset AD (LOAD) not only experienced no adverse events but also demonstrated significant cognitive preservation and biomarker improvement, with the key AD plasma biomarker pTau217 decreasing by up to 47%. In contrast, two men with early-onset AD (EOAD) did not show clear benefit. This study represents the longest clinical exploration of 40Hz sensory stimulation therapy to date and provides important evidence for targeted AD intervention.

This research is an extension of an earlier MIT clinical trial (NCT04055376) conducted in 2020, which was interrupted after three months due to the pandemic despite preliminary observations of cognitive and brain volume preservation in 15 mild AD patients.[1]
In this open-label extension study, five volunteers chose to continue using the device—a home-based system consisting of a two-foot square LED panel and speakers that synchronously deliver 40Hz light pulses and sound, paired with a tablet providing entertainment content to facilitate daily one-hour home sessions. The five patients had distinct baseline profiles: three women with LOAD (onset age 72-87, all APOE E3/E3) and two men with EOAD (onset age 54-61, one APOE E3/E4). All initially met criteria for mild AD (MMSE 22-26, CDR global score of 1), and their plasma pTau217 levels were all above the 6.8 pg/mL AD diagnostic threshold.
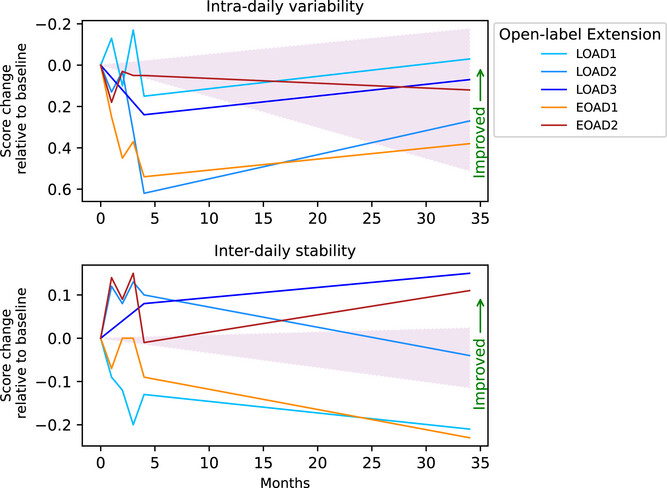
To comprehensively assess efficacy, the research team conducted multi-dimensional testing over 30 months (including the initial trial phase): electroencephalography (EEG) monitored the brain's "entrainment" to the stimulation; magnetic resonance imaging (MRI) measured volumes of AD-vulnerable brain regions like the hippocampus and amygdala; actigraphy tracked sleep and circadian rhythms; and five standard assessments including the Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), and Functional Assessment Scale (FAS) evaluated cognitive and daily function. Plasma pTau217 levels were measured using S-PLEX technology. To account for individual variability, researchers also matched the participants against thousands of untreated AD patients from three major databases—the National Alzheimer's Coordinating Center (NACC), Alzheimer's Disease Neuroimaging Initiative (ADNI), and Longitudinal Early-onset Alzheimer's Disease Study (LEADS)—based on age, sex, and initial cognitive scores.
The results revealed a clear "subtype divergence." The three women with LOAD responded significantly to treatment. Their EEGs showed 40Hz power increases of 109%, 164%, and 113% from baseline at 30 months, with stable signal strength in the occipital (vision-related) and some frontal (auditory-related) regions, indicating their brains continued to effectively "follow" the stimulation rhythm. In contrast, the two men with EOAD showed 40Hz power reductions to 38% and 32% of baseline, with nearly vanished EEG responses to the stimulus. Cognitively, the decline in MMSE, CDR, and FAS scores for the LOAD women was much slower than in matched controls. For instance, their annual MMSE score decline was 0.8-1.2 points less than matched LOAD patients from the databases. Improvements in CDR (dementia severity) and FAS (daily function) reached statistical significance (p-values 0.02, 0.02, and 0.01, respectively). Conversely, the EOAD men showed no significant difference in cognitive scores compared to controls, with some indicators declining even faster.
More crucially, biomarker changes were observed: in the two LOAD women who provided plasma samples, pTau217 levels decreased by 47% (from 27.6 pg/mL to 14.5 pg/mL) and 19.4% (from 9.78 pg/mL to 7.88 pg/mL) after two years. Even after correction for total plasma protein, the reductions were 54.9% and 19.2%. pTau217 is the first plasma-based diagnostic biomarker for AD approved by the FDA last year and is highly correlated with brain tau tangle burden. This reduction via a non-pharmacological intervention is the first such report in human AD research, suggesting 40Hz stimulation may directly impact the AD pathological process—echoing findings from prior mouse studies where 40Hz gamma oscillations reduced amyloid-beta and tau accumulation.
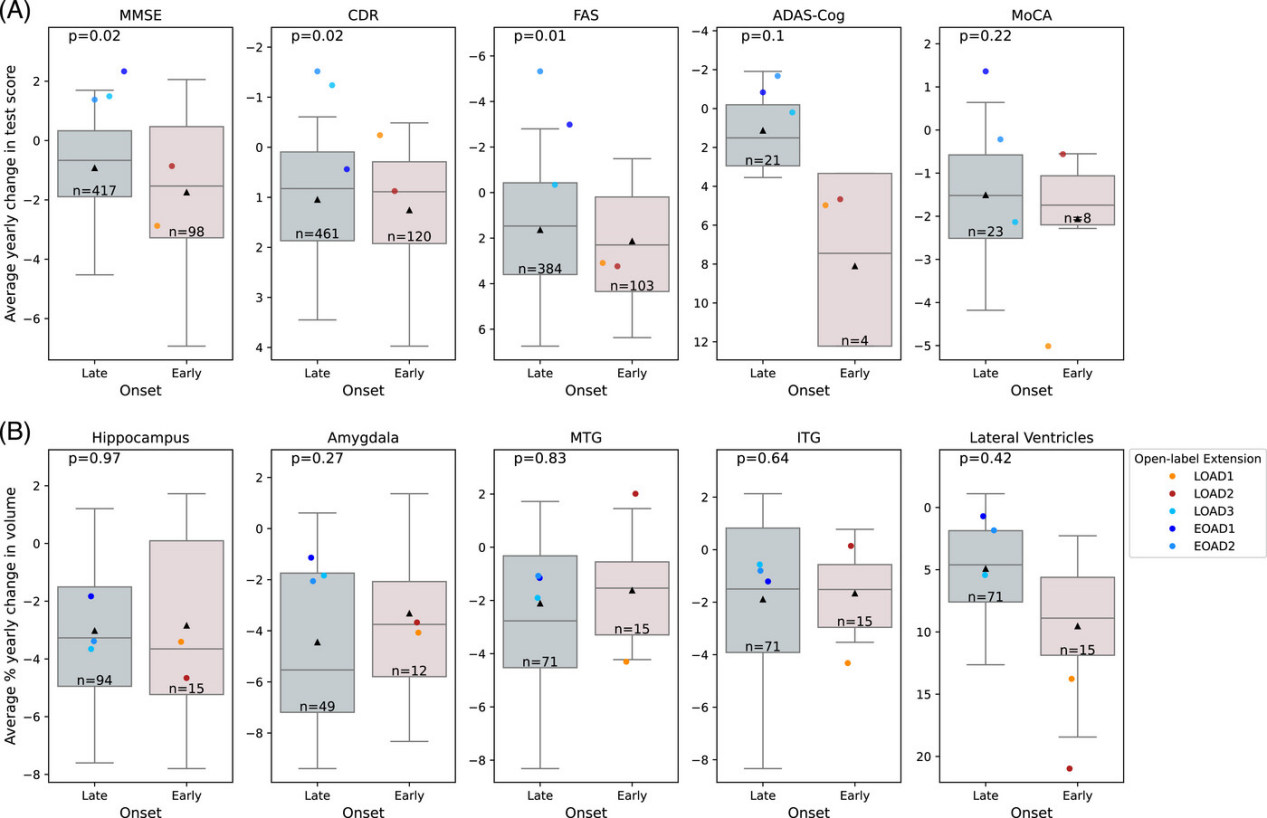
Safety and feasibility were also notable: no serious adverse events were reported over two years. Only one initial control participant experienced brief anxiety (resolved in 10 minutes) and another reported initial fatigue (adapted within one month). Patients successfully completed treatments at home with good compliance, providing a practical foundation for long-term intervention. However, the study has limitations: MRI brain volume analysis showed no significant differences (possibly because AD-related atrophy progresses slowly over two years, requiring larger samples for validation), and the divergence between EOAD and LOAD patients may be influenced by underlying pathology—EOAD typically involves more aggressive pathology and broader neural network damage, potentially requiring a higher threshold for sensory stimulation response.
Currently, Cognito Therapeutics, the MIT spin-off developing this therapy, is conducting a nationwide clinical trial. The research team has also begun a new exploration: recruiting individuals over 55 with normal memory but a family history of AD to test the preventive potential of 40Hz stimulation, aiming to block pathological progression before disease onset. [2] "This study demonstrates that long-term, at-home use of 40Hz audiovisual stimulation is safe and feasible, and for patients with late-onset AD in particular, it may become a new option to slow cognitive decline," said the study's lead author, Massachusetts General Hospital neurologist Diane Chan. "The next step is to identify more predictors of treatment response so this non-invasive therapy can precisely benefit more patients."
[1] Diane Chan Brennan L. Jackson, Ho-Jun Suk, et al. Gamma sensory stimulation in mild Alzheimer's dementia: An open-label extension study, Alzheimer's & Dementia, (2025). DOI: 10.1002/alz.70792.
https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.70792
[2]GabrielledeWeck, New trial to test brain wave stimulation as Alzheimer' s preventative, The Picower Institute. August 25, 2020.
https://picower.mit.edu/news/new-trial-test-brain-wave-stimulation-alzheimers-preventative




