New Breakthrough in Flexible Neural Chips: 40 Hz Stimulation Synchronously Regulates Dopamine and Cognition in Alzheimer's Mice
GemPagesResearch into Alzheimer's disease (AD) treatment is entering a new era that combines precise modulation with real-time feedback. A long-standing critical bottleneck in understanding therapeutic mechanisms has been how to instantly "read" the dynamic changes in neurochemical substances while simultaneously intervening in the brain. In July 2025, a team from the Chinese Academy of Sciences published groundbreaking research in Nature Communications, successfully developing a flexible multifunctional neural chip named NeuroRevive-FlexChip and validating its efficacy in AD model mice [1]. This study not only achieved, for the first time, the simultaneous application of 40 Hz hippocampal electrical stimulation and in situ dopamine monitoring but also revealed the complete chain of evidence showing how stimulation rapidly "reboots" the dopamine system, suppresses abnormal neural synchronization, and thereby improves cognition. It provides revolutionary tools and insights for the closed-loop, precision treatment of AD.
The Challenge and the Breakthrough: From "Unidirectional Intervention" to "Interactive Modulation"
Traditional brain electrical stimulation techniques, while capable of modulating neural networks, function like a "black box"—we cannot determine what instantaneous effect it has on key neurotransmitters (such as dopamine) at the very same time and location the intervention is applied. This "output-only" mode severely limits our understanding of the mechanisms and optimization of treatment protocols.
The development of the NeuroRevive-FlexChip aims to break this deadlock. The research team innovated from the material source, employing flexible parylene as the substrate. Its mechanical properties are highly compatible with soft brain tissue, significantly reducing inflammation and damage from long-term implantation and laying the groundwork for stable, long-term observation and intervention. The core breakthrough of the chip lies in its integrated "three-in-one" design: 1) a modulation unit capable of delivering precise 40 Hz electrical stimulation; 2) a sensing unit for highly sensitive detection of extracellular dopamine concentration; and 3) an electrophysiological unit for recording the electrical activity of neuronal populations.
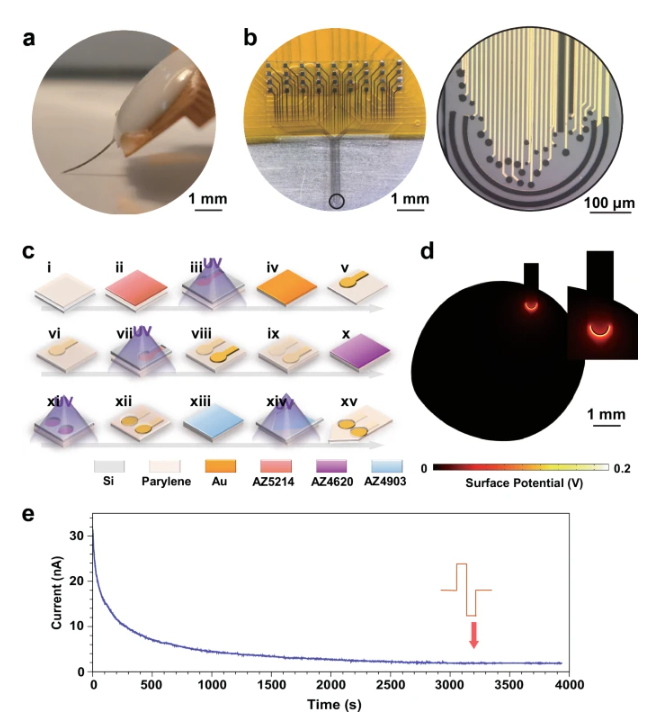
Figure 1: Structure and design principles of the NeuroRevive-FlexChip.
Core Findings: How 40 Hz Stimulation 'Reboots' the Diseased Brain
Using this chip to conduct experiments in the hippocampal CA1 region of APP/PS1 transgenic AD mice, the study obtained several sequential key pieces of evidence, from molecular to behavioral levels:
1. Rapid 'Rebooting' of Dopamine Release
Real-time monitoring by the chip captured a clear phenomenon: upon applying 40 Hz stimulation, extracellular dopamine levels rapidly increased within seconds. Although levels subsequently declined due to metabolism, they showed a trend of rising again during the inter-stimulus intervals, forming a dynamic "reboot" pattern. This directly proves that 40 Hz stimulation can rapidly and transiently activate the dopamine system, which is functionally impaired in the AD brain.
2. Suppression of Pathological Neural Synchrony
A typical pathological feature of the AD brain is the excessive synchronization of neuronal firing. Chip recordings revealed that the baseline firing frequency in the hippocampus of AD mice was much higher than in normal mice. After 40 Hz stimulation, the abnormally elevated average firing frequency was significantly suppressed, with reductions of up to 94%. Furthermore, the peak dopamine release induced by stimulation correlated with "burst" firing of neurons, while subsequent dopamine levels corresponded to more stable "tonic" firing. This suggests that stimulation may guide the neural network from a chaotic state towards a more stable, orderly operating mode via the dopamine pathway.
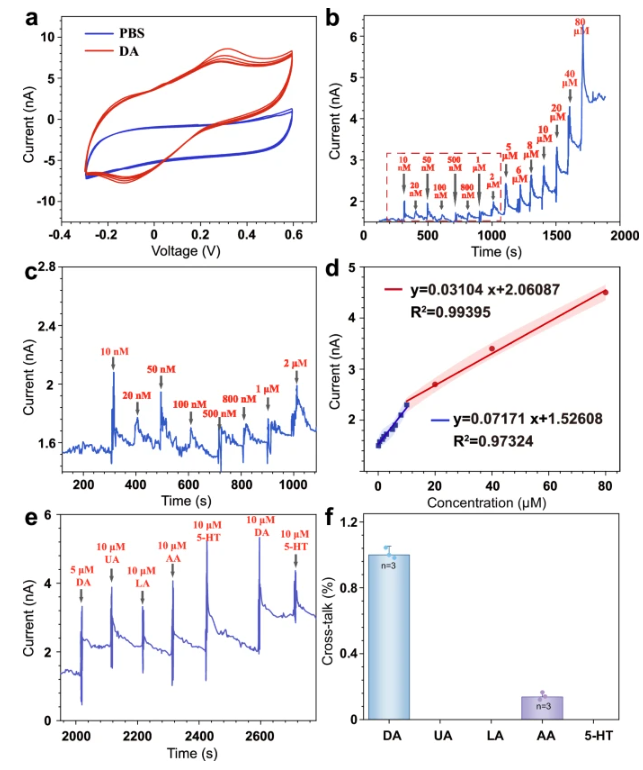
Figure 2: Dopamine dynamics and neural activity changes induced by 40 Hz stimulation.
3. Improvement in Spatial Memory and Pathology
At the behavioral level, treated AD mice showed a significantly increased percentage of correct spontaneous alternations in the Y-maze test, indicating improved spatial working memory. At the molecular level, researchers observed reduced Aβ42 plaque deposition in the mouse hippocampus and a shift in microglial morphology towards a more protective, phagocytic phenotype. These findings provide a potential pathophysiological basis for the behavioral improvements.
Technological Foundation: Outstanding Performance Ensures Reliability of Discoveries
The reliability of these biological findings is rooted in the excellent physicochemical performance of the NeuroRevive-FlexChip. The specially coated microelectrodes exhibit extremely high electrochemical detection sensitivity, with a detection limit for dopamine as low as 0.1 micromolar, and effectively discriminate against interfering substances like ascorbic acid. Simultaneously, their impedance is four orders of magnitude lower than that of bare electrodes, with a significantly increased charge storage capacity. This ensures stimulation efficiency while minimizing potential damage to brain tissue.
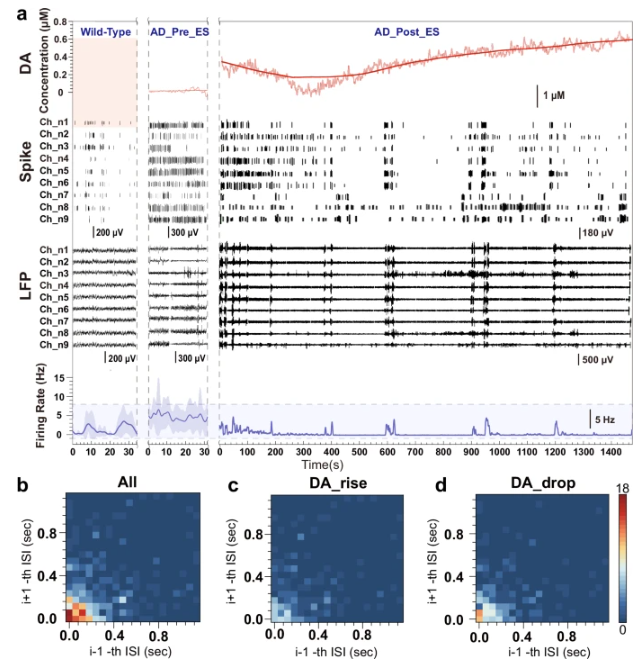
Figure 3: Key data on the chip's electrochemical performance and stimulation efficiency.
Future Outlook: Towards an Adaptive 'Neural Pacemaker'
The profound significance of this study lies in the fact that it not only reports a new therapeutic effect but, more importantly, provides a set of "seeing-through" tools capable of revealing how this effect occurs. For the first time in a living brain, the NeuroRevive-FlexChip provides real-time, causally-linked empirical evidence connecting 40 Hz gamma oscillation stimulation, dopaminergic system modulation, and neural network stability, filling a critical gap in the neurochemical evidence chain for "frequency therapy."
Looking ahead, this technology outlines a clear path for the evolution of neuromodulation therapies from "open-loop" to "closed-loop" systems. Next-generation devices based on this principle hold promise for developing into intelligent "adaptive neural pacemakers": they could automatically assess disease status based on dopamine levels or abnormal electrical activity signals monitored in real-time by the chip and trigger electrical stimulation with personalized parameters. This would enable truly integrated management of AD pathology progression, combining real-time monitoring, intelligent diagnosis, and precise intervention.
Of course, numerous challenges remain in translating this from mice to human clinical applications, including validation of long-term biosafety, wireless power supply, data transmission, and confirmation of efficacy in the more complex human brain environment. Nevertheless, this research undoubtedly marks an important starting point. Through the deep integration of flexible electronics technology and neuroscience, we have illuminated an innovative path with significant potential for ultimately conquering complex brain disorders like Alzheimer's disease.
[1]Lv, S., Mo, F., Xu, Z. et al. Integrated dopamine sensing and 40 Hz hippocampal stimulation improves cognitive performance in Alzheimer’s mouse models. Nat Commun 16, 5948 (2025).
https://www.nature.com/articles/s41467-025-60903-1




