How Does 40Hz Sensory Stimulation Improve Spatial Navigation in Alzheimer's Model Mice?
GemPagesAlzheimer's disease (AD) is one of the most prevalent neurodegenerative disorders, characterized by early hippocampal dysfunction and abnormal neural activity. The "slow gamma rhythm" (25-55 Hz) is known to play a crucial role in coordinating information transfer between hippocampal subregions, particularly the transmission of spatial and memory-related information from the CA3 to the CA1 area.
Recent studies have found that 40Hz rhythmic sensory stimulation (flicker), also termed "multisensory gamma stimulation," can reduce Aβ deposition, modulate the brain's immune microenvironment, and improve cognitive function. However, little is known about how it specifically affects memory-related neural coding processes.

A study published on April 22, 2025, in PNAS by a neuroscience team at the Georgia Institute of Technology (Georgia Tech) provides an in-depth investigation into the effects of 40Hz sensory stimulation on "prospective neural coding" and CA3–CA1 circuit communication in the brains of Alzheimer's model mice.[1] By recording the activity of thousands of neurons in the hippocampus, the research team aimed to uncover:
1. Whether 40Hz flicker enhances information synchronization between neurons.
2. Whether it improves the ability to encode "future paths" during memory processes.
3. Whether these changes in neural activity are genuinely linked to more efficient behavioral performance.
Experimental Design: High-throughput Electrophysiology + Virtual Reality Navigation Task
The study used 5XFAD mice (a widely adopted AD model exhibiting Aβ deposition and memory deficits) and established a virtual reality (VR) spatial navigation system:
1. Mice were head-fixed on a spherical treadmill.
2. A circular virtual track featured reward points marked by visual cues.
3. Mice received a sweet milk reward by licking at the correct location.
4. Silicon probes were used to simultaneously record the activity of thousands of neurons in the CA3 and CA1 regions.
5. The experimental group received 40Hz periodic stimulation, while the control group received pseudo-random frequency stimulation.
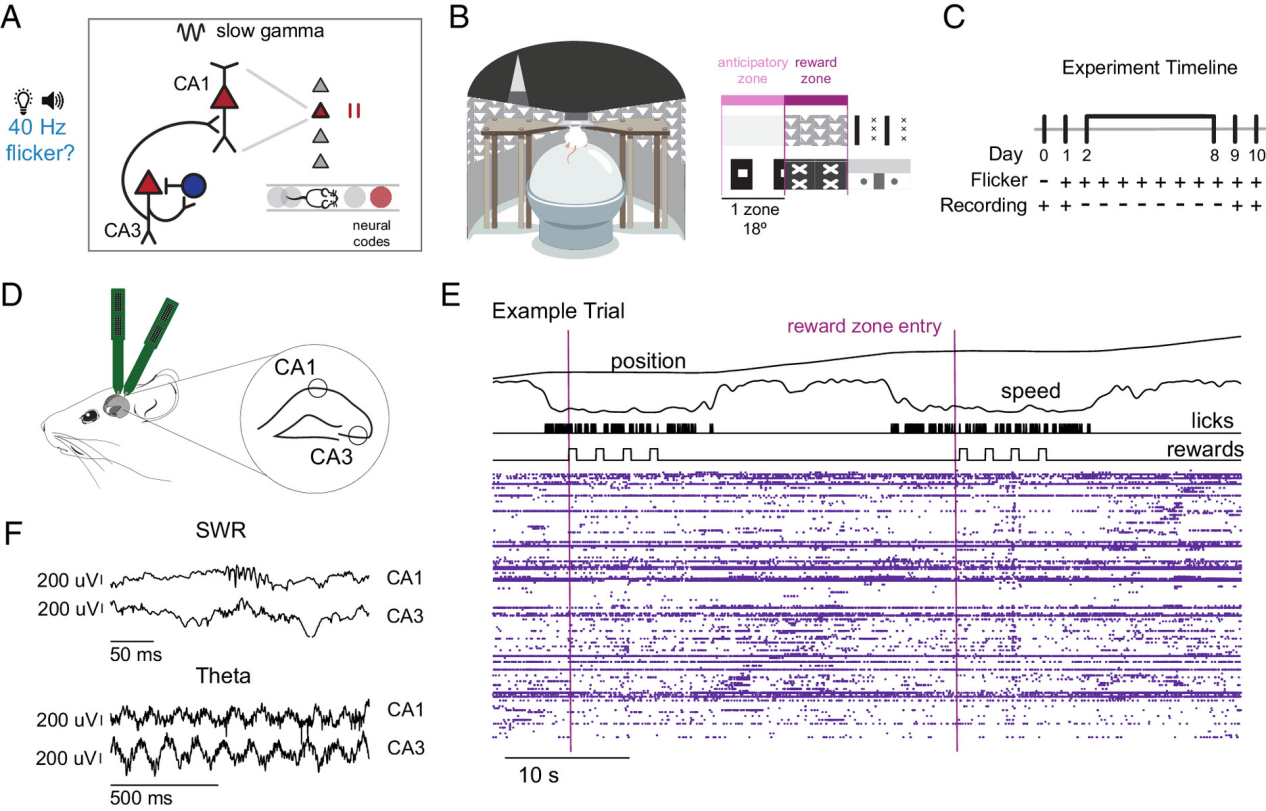
Figure 1: Timeline of a goal-directed spatial navigation task performed by 5XFAD mice before and after an 8-day regimen of 40 Hz flicker stimulation.
Finding 1: 40Hz Flicker Enhances "Slow Gamma Coupling" Between CA3 and CA1
During navigation, mice receiving 40Hz flicker stimulation showed significantly enhanced slow gamma phase coupling between CA3 and CA1. This means that firing in CA3 neurons was more likely to generate synchronous responses in CA1, improving the "communication quality" between these two regions.
This coordination primarily occurred during the behavioral task, not just during stimulation itself, suggesting a "carry-over effect" or plasticity change. This provides strong experimental evidence for the supportive role of the slow gamma rhythm in memory encoding.
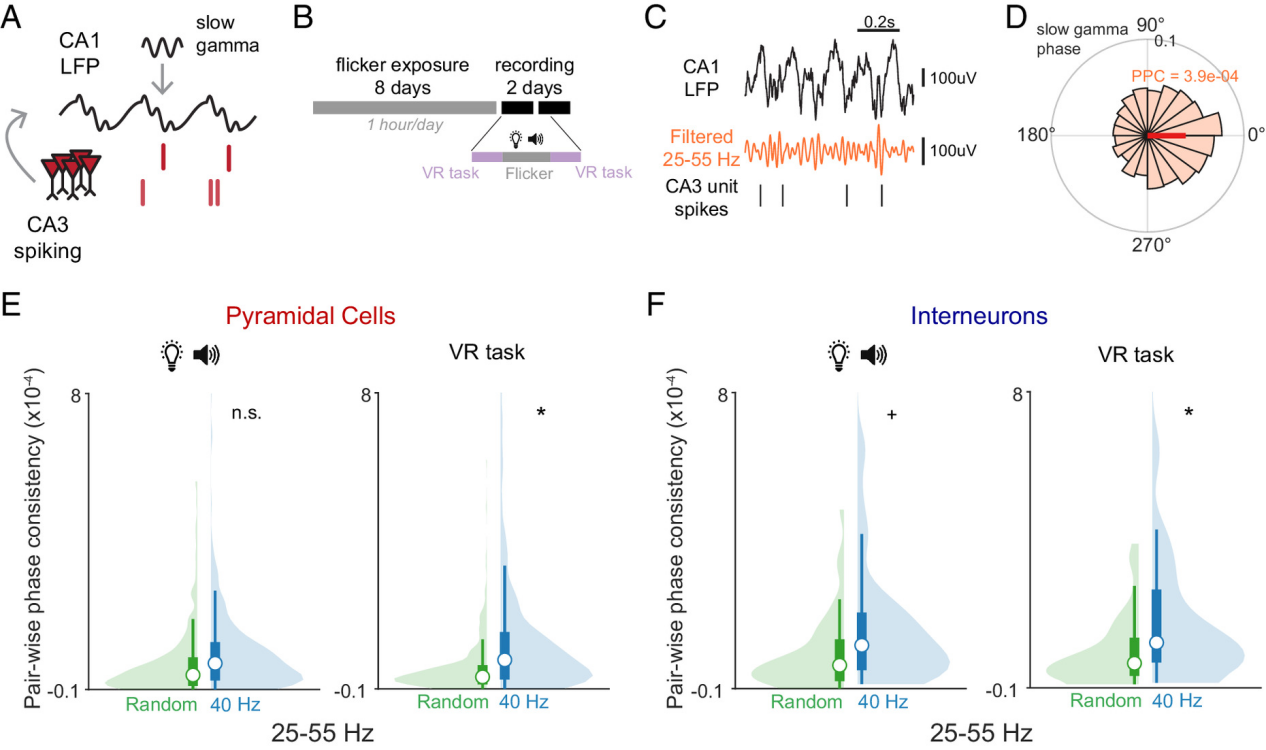
Figure 2: Enhanced spike-phase coupling between CA3 and CA1 following 40 Hz stimulation.
Finding 2: Enhanced "Prospective Neural Coding" Ability to Predict Future Locations
"Prospective coding" refers to the phenomenon, observed under hippocampal theta rhythm, where neuronal populations do not merely encode the current location but activate in sequence, representing the animal's potential future path.
The study found that this encoding of future paths was markedly enhanced in the 40Hz stimulation group: before entering the reward zone, neural activity was more inclined to represent "the next area to be visited."
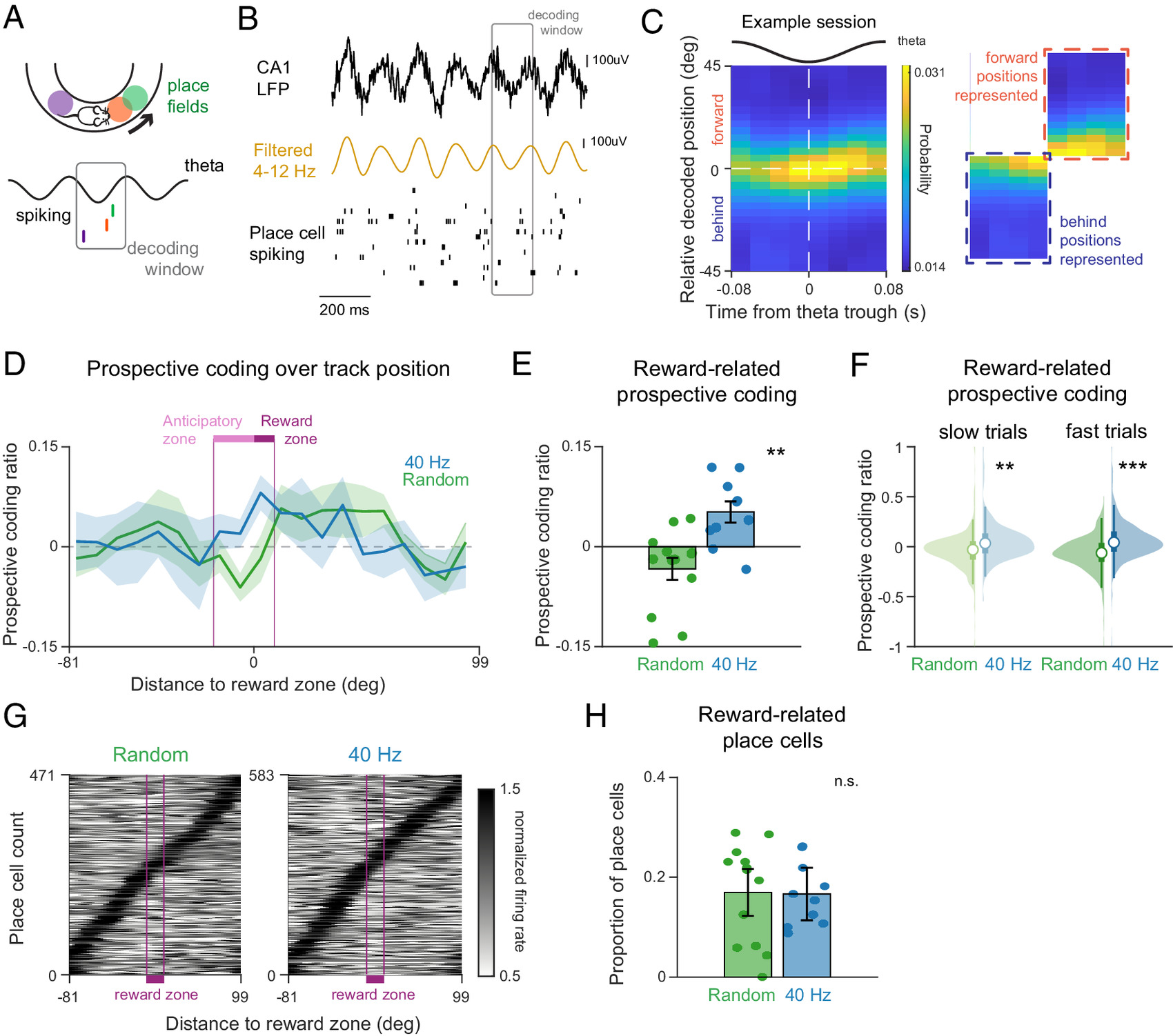
Figure 3: 40 Hz flicker stimulation enhances prospective coding during theta oscillations.
Even during Sharp-Wave Ripples (SWRs)—hippocampal replay events occurring during quiet behavior—reactivation more strongly favored future trajectories. In contrast, the control group showed more retrospective coding (replaying past paths).
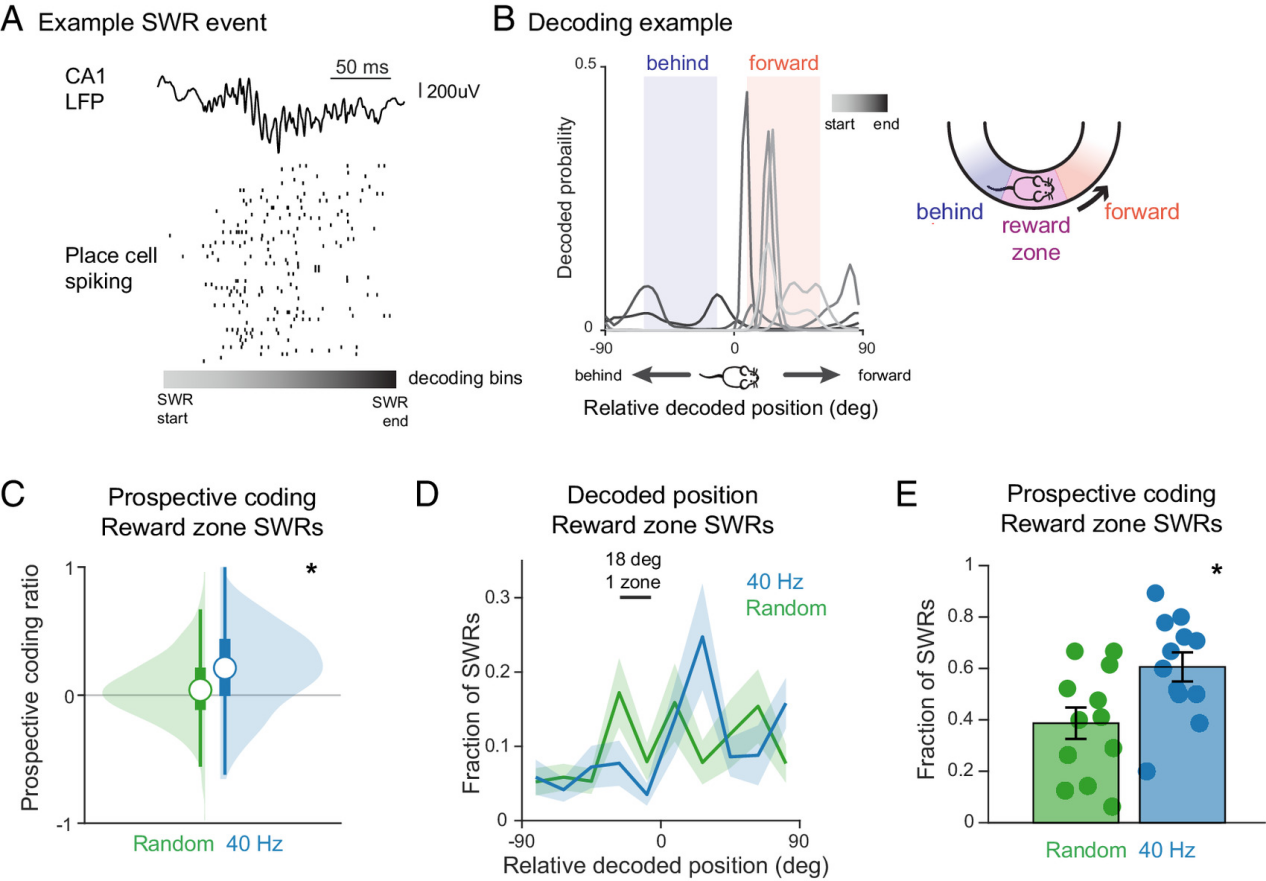
Figure 3: 40 Hz flicker stimulation enhances prospective coding during SWRs.
Finding 3: Changes in Neural Coding Directly Correlate with Behavioral Performance
The researchers further categorized navigation behavior into "efficient (fast and accurate)" and "inefficient (slow or erroneous)" types.
They found that the strength of prospective coding was highly correlated with navigation efficiency: during fast running, an animal's neurons showed a stronger bias toward activating future path codes. Mice that licked more actively just before reward delivery also exhibited stronger prospective coding.
Importantly, this enhancement was not driven by running speed itself but was highly associated with the animal's "engagement" in and "anticipation" of the task.
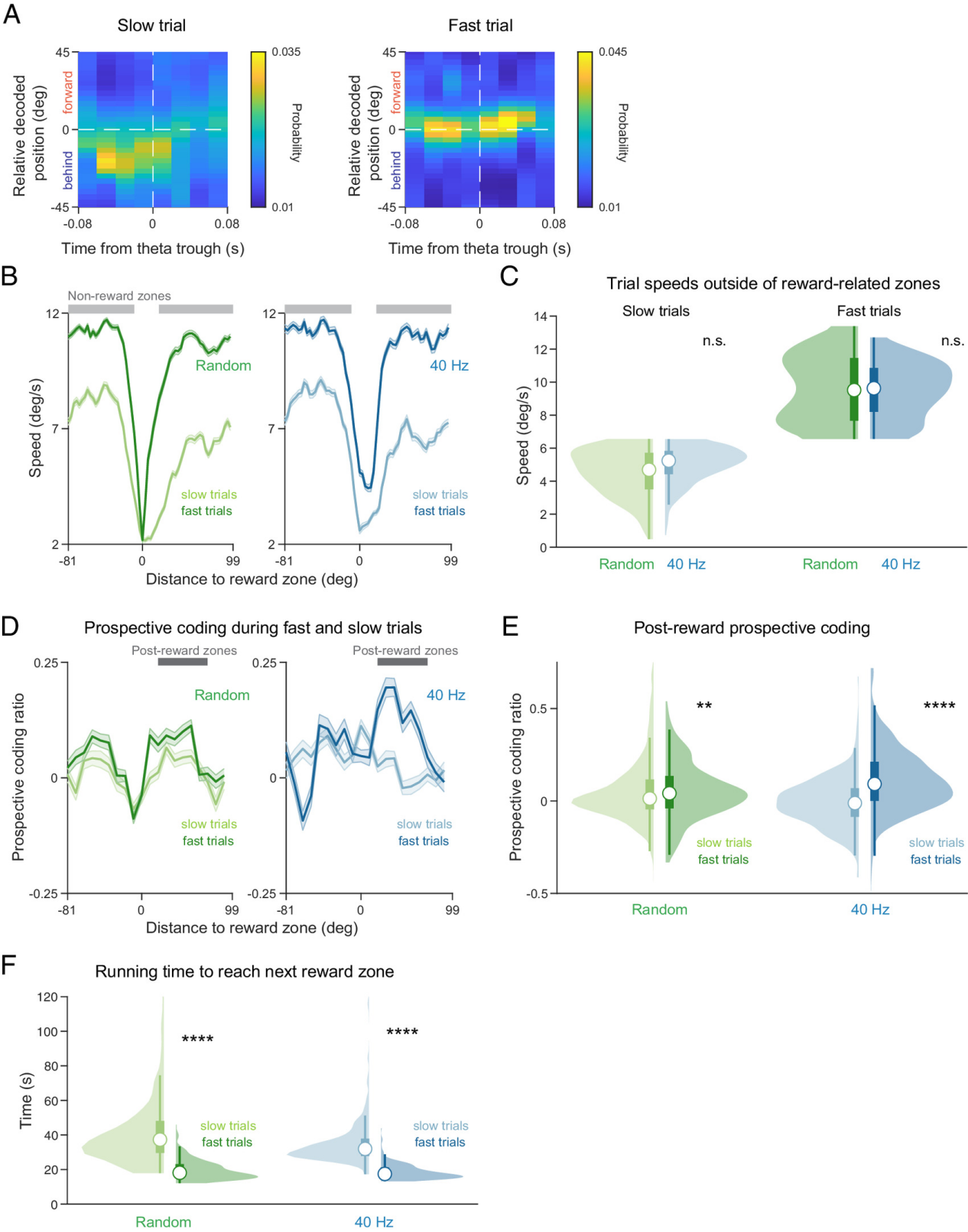
Figure 3: 40 Hz flicker stimulation enhances prospective coding during Sharp-Wave Ripples (SWRs).
Summary and Implications: Can Rhythmic Stimulation "Train" Memory Circuits?
This research not only provides robust neural circuit-level evidence for the potential therapeutic mechanisms of "40Hz sensory stimulation" but also suggests that memory is not just about "storing the past"—it also involves predicting the future. By using external stimulation to "mimic" brain rhythms, it may be possible to remodel memory pathways. Especially for early intervention in cognitive disorders like AD, rhythmic neuromodulation could be a promising breakthrough.
[1] Abigail L. Paulson, Lu Zhang, Ashley M. Prichard, et al.40 Hz sensory stimulation enhances CA3–CA1 coordination and prospective coding during navigation in a mouse model of Alzheimer’s disease. PNAS. April 22, 2025. 122 (17) e2419364122
https://www.pnas.org/doi/10.1073/pnas.2419364122




